Primary photochemical mechanisms
Borate Salts as Coinitiators in Photoinitiating Systems for Laser Imaging
In this paper, visible light photoinitiating systems are developed for Free Radical Photopolymerization FRP. The use of borate salts as coinitiators was found to yield high rate of polymerization as well as high final conversion when combined with a dye abosrbing in the UV-visible range. The mechanism of reaction was investigated by laser flash photolysis. The mechanism is discussed in terms of photoinduced electron transfer and fast dissociation of borate radical.
Personnel: Allonas X.; Ibrahim A.; Ley C.; et al.
Source: JOURNAL OF PHOTOPOLYMER SCIENCE AND TECHNOLOGY Volume: 24 Issue: 5 Pages: 531-534 Published: 2011
Pyrromethene derivatives in three-component photoinitiating systems for free radical photopolymerization
1,3,5,7,8-pentamethyl pyrromethene difluoroborate complex (HMP) and 2,6-diethyl-8-phenyl-1,3,5,7-tetramethylpyrromethene difluoroborate complex (EPP) were used to initiate the polymerization of a diacrylate in a two- and a three-component photoinitiating system (PIS), together with an amine (ethyl-4-dimethylaminobenzoate, EDB) and triazine A (2-(4-methoxyphenyl)-4,6-bis(trichloromethyl)-1,3,5-triazine, TA) as coinitiators. For both pyrromethene dyes, the highest conversion was achieved with the three-component PIS. As these dyes have high-fluorescence quantum yields, steady state and time-resolved techniques were used to study the possible fluorescence quenching by the amine and the triazine, as well as laser flash photolysis to investigate the electron transfer process that occurs in these PIS from either the singlet or triplet excited states. The electron transfer reaction is evidenced by using time-resolved photoconductivity. Experiments show that the main interaction between the dye and both coinitiators is through its excited singlet state and the process is more efficient when TA is present. The beneficial effect noted when both coinitiators are used in a three-component system is ascribed to secondary reactions between the coinitiators and intermediates that lead to the generation of higher amount of initiating species and the recovery of the initial dye.
Contact: Xavier Allonas
J. Polym. Sci., Part A : Polym. Chem., 48, 2594-2603 (2010)
Near UV–visible light induced cationic photopolymerization reactions: A three component photoinitiating system based on acridinedione/silane/iodonium salt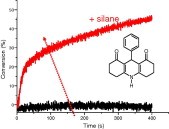
Acridinediones (AD) are attractive dyes exhibiting a strong absorption at about 400 nm. When used in the presence of a silane and an iodonium salt, they efficiently initiate the free radical promoted cationic photopolymerization of an epoxide under air and upon a xenon lamp, a laser diode or sunlight exposure. The mechanism analyzed by ESR and laser flash photolysis is discussed and opens a new way for the design of such three-component systems.
Contact: Xavier Allonas
Representative publication : European Polymer Journal, Vol.46, 2138–2144 (2010)
Pentacyano-N,N-Dimethylaniline in the Excited State. Only Locally Excited State Emission, in Spite of the Large Electron Affinity of the Pentacyanobenzene Subgroup
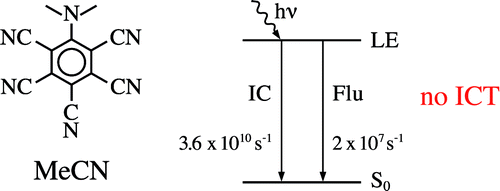 Pentacyano-N,N-dimethylaniline (PCDMA) does not undergo an intramolecular charge transfer (ICT) reaction, even in the strongly polar solvent acetonitrile (MeCN), in clear contrast to 4-(dimethylamino)benzonitrile (DMABN). Within the twisted ICT (TICT) model, this is unexpected, as the electron affinity of the pentacyanobenzene moiety of PCDMA is much larger than that of the benzonitrile subgroup in DMABN. According to the TICT model, the energy of the ICT state of PCDMA would be 2.05 eV (
Pentacyano-N,N-dimethylaniline (PCDMA) does not undergo an intramolecular charge transfer (ICT) reaction, even in the strongly polar solvent acetonitrile (MeCN), in clear contrast to 4-(dimethylamino)benzonitrile (DMABN). Within the twisted ICT (TICT) model, this is unexpected, as the electron affinity of the pentacyanobenzene moiety of PCDMA is much larger than that of the benzonitrile subgroup in DMABN. According to the TICT model, the energy of the ICT state of PCDMA would be 2.05 eV (![]() 16550 cm−1) lower than that of DMABN, on the basis of the reduction potentials E(A−/A) of pentacyanobenzene (−0.29 V vs saturated calomel electrode (SCE)) and benzonitrile (−2.36 V vs SCE), more than enough to compensate for the decrease in energy of the locally excited (LE) state of PCDMA (E(S1) = 19990 cm−1) relative to that of DMABN (E(S1) = 29990 cm−1). This absence of a LE → ICT reaction shows that the TICT hypothesis does not hold for PCDMA in the singlet excited state, similar to what was found for DMABN, N-phenylpyrrole, and their derivatives. In this connection, the six dicyano-substituted dimethylanilines are also discussed. The energy gap ΔE(S1,S2) between the two lowest singlet excited states is, at 7170 cm−1 for PCDMA in MeCN, considerably larger than that for DMABN (2700 cm−1 in n-hexane, smaller in MeCN). The absence of ICT is therefore in accord with the planar ICT (PICT) model, which considers a sufficiently small ΔE(S1,S2) to be an important condition determining whether an ICT reaction will take place. The fluorescence quantum yield of PCDMA is very small: Φ(LE) = 0.0006 in MeCN at 25 °C, predominantly due to LE → S0 internal conversion (IC), as the intersystem crossing yield Φ(ISC) is practically zero (<0.01). From the LE fluorescence decay time of 27 ps for PCDMA in MeCN at 25 °C, a radiative rate constant kf(LE) = 2 × 107 s−1 results, comparable to the kf(LE) of DMABN (6.5 × 107 s−1) and 2,4,6-tricyano-N,N-dimethylaniline (TCDMA) (1.2 × 107 s−1) in this solvent, but clearly larger than the k′f(ICT) = 0.79 × 107 s−1 of DMABN in MeCN. The IC reaction with PCDMA in MeCN at room temperature, with a rate constant kIC of 3.6 × 1010 s−1, is much faster than with TCDMA (25 × 107 s−1) and DMABN (1.3 × 107 s−1, in n-hexane). This is connected with the nonzero (37°) amino twist angle of PCDMA, which leads to a decrease of the effective LE−S0 energy gap. The femtosecond excited state absorption (ESA) spectra of PCDMA in MeCN at 22 °C are similar to the LE ESA spectra of TCDMA and DMABN and are therefore attributed to the LE state, confirming that an ICT reaction does not occur. The decay of the LE ESA spectra of PCDMA is single exponential, with a decay time of 22 ps, in reasonable agreement with the LE fluorescence decay time of 27 ps at 25 °C. The spectra decay to zero, showing that there is no triplet or other intermediate.
16550 cm−1) lower than that of DMABN, on the basis of the reduction potentials E(A−/A) of pentacyanobenzene (−0.29 V vs saturated calomel electrode (SCE)) and benzonitrile (−2.36 V vs SCE), more than enough to compensate for the decrease in energy of the locally excited (LE) state of PCDMA (E(S1) = 19990 cm−1) relative to that of DMABN (E(S1) = 29990 cm−1). This absence of a LE → ICT reaction shows that the TICT hypothesis does not hold for PCDMA in the singlet excited state, similar to what was found for DMABN, N-phenylpyrrole, and their derivatives. In this connection, the six dicyano-substituted dimethylanilines are also discussed. The energy gap ΔE(S1,S2) between the two lowest singlet excited states is, at 7170 cm−1 for PCDMA in MeCN, considerably larger than that for DMABN (2700 cm−1 in n-hexane, smaller in MeCN). The absence of ICT is therefore in accord with the planar ICT (PICT) model, which considers a sufficiently small ΔE(S1,S2) to be an important condition determining whether an ICT reaction will take place. The fluorescence quantum yield of PCDMA is very small: Φ(LE) = 0.0006 in MeCN at 25 °C, predominantly due to LE → S0 internal conversion (IC), as the intersystem crossing yield Φ(ISC) is practically zero (<0.01). From the LE fluorescence decay time of 27 ps for PCDMA in MeCN at 25 °C, a radiative rate constant kf(LE) = 2 × 107 s−1 results, comparable to the kf(LE) of DMABN (6.5 × 107 s−1) and 2,4,6-tricyano-N,N-dimethylaniline (TCDMA) (1.2 × 107 s−1) in this solvent, but clearly larger than the k′f(ICT) = 0.79 × 107 s−1 of DMABN in MeCN. The IC reaction with PCDMA in MeCN at room temperature, with a rate constant kIC of 3.6 × 1010 s−1, is much faster than with TCDMA (25 × 107 s−1) and DMABN (1.3 × 107 s−1, in n-hexane). This is connected with the nonzero (37°) amino twist angle of PCDMA, which leads to a decrease of the effective LE−S0 energy gap. The femtosecond excited state absorption (ESA) spectra of PCDMA in MeCN at 22 °C are similar to the LE ESA spectra of TCDMA and DMABN and are therefore attributed to the LE state, confirming that an ICT reaction does not occur. The decay of the LE ESA spectra of PCDMA is single exponential, with a decay time of 22 ps, in reasonable agreement with the LE fluorescence decay time of 27 ps at 25 °C. The spectra decay to zero, showing that there is no triplet or other intermediate.
Personnel: Zachariasse Klaas A.; Allonas X. et al.
Source: JOURNAL OF PHYSICAL CHEMISTRY A, 2010, Volume: 114 Issue: 50 Pages: 13031-13039
Photoinitiation Mechanism of Free Radical Photopolymerization in the Presence of Cyclic Acetals and Related Compounds
The behavior of six cyclic acetals and related compounds in the photoinitiation step of a radical photopolymerization was investigated. As shown by the photopolymerization kinetic data obtained from FTIR spectroscopy, most of them are efficient coinitiators in the presence of benzophenone (BP) with efficiencies close to a reference amine coinitiator (ethyl dimethylaminobenzoate, EDB). Laser flash photolysis and ESR spin trapping technique were used to study the photochemical mechanisms of the production of initiating radicals and explain the differences in reactivity.
Personnel: Allonas X.; et al.
Source: JOURNAL OF POLYMER SCIENCE PART A-POLYMER CHEMISTRY, 2010, Volume: 48 Issue: 24 Pages: 5758-5766
Investigation of barton esters as radical photoinitiators
Different photoinitiators based on Barton thiohydroxamic esters, O-acyl-N-hydroxy-pyridine-2(1H)-thione and O-acyl-N-hydroxy-thiazole-2(3H)-thione derivs., were tested in photopolymn. reactions through time-resolved FTIR spectroscopy. Good rates of polymn. and final monomer conversion were obtained for some compds. The excited state processes, investigated by time resolved absorption spectroscopy, lie on a fast singlet state cleavage leading to thiyl and alkyl radicals. Both the pyridine-2(1H)-thiyl and alkyl radicals are able to initiate a polymn., in contrast with the thiazole-2(3H)-thiyl radical. A triplet state is obsd. for some derivs. Computational studies help to describe the excited state properties and show a strong difference in the spin localization in the formed thiyl initiating radicals.
Personnel : Céline Dietlin, Xavier Allonas, Fabrice Morlet-Savary, Jean-Pierre Fouassier
Publication significative : J. of Appl. Polym. Sci., 109(2), 825-833 (2008)
Photochemistry of Naphthalimide Photoacid Generators
The photophysical properties of a series of 1,8-naphthalimide photoacid generators were studied by steady state fluorescence and phosphorescence spectroscopy. Emission and excitation anisotropies, triplet quantum yields in polar and nonpolar solvent and photoacid generation were evaluated. The singlet excited state exhibits a low polarity and is strongly deactivated by an efficient intersystem crossing process. In protic solvent, a homolytic singlet cleavage of the N−O bond occurs and leads to the acid production. The existence of a triplet state close to the singlet state was clearly evidenced. The presence of close singlet excited states is supported by fluorescence anisotropy and picosecond laser spectroscopy experiments. Results of DFT calculations well confirm the experimental contentions and yield important information about the cleavage process involved in such compounds.
Contact: Xavier Allonas
J. Phys. Chem. A, 112, 3879-3885 (2008)
Photochemistry and photophysics of a morpholino methylthio phenyl ketone: A steady-state, picosecond pump-probe laser spectroscopy and molecular modeling investigation
The photophysics of 2-methyl-1-[4-(methylthio) phenyl]-2-(4-morpholinyl)-1-propanone TPMK, compared to that of two reference compounds (2-methyl-1-phenyl-2-(4-morpholinyl)-1-propanone and 1-[4-(methylthio)phenyl]-ethanone), was studied by means of absorption spectroscopy, phosphorescence and time-resolved absorption spectroscopy. A four-level kinetic scheme has been proposed for TPMK as an explanation for the observed excited state processes. A strong solvent effect has been noticed upon the excited state lifetimes. Modeling calculations help to describe the excited state properties.
Contact: Xavier Allonas
Publication significative : J. Photochem. Photobiol., A : Chem., 197, 342-350 (2008)
Two-photon absorption and polymerization ability of intramolecular energy transfer based photoinitiating systems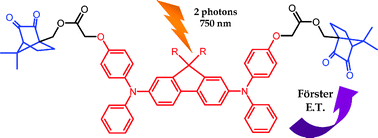
We design a new photoinitiating system where the two-photon absorption of a 2,7 bisaminofluorene moiety leads to the photoactivation of a camphorquinone subunit through a Förster-type intramolecular energy transfer: the application to a two-photon polymerization reaction is demonstrated.
Contact: Xavier Allonas
Publication significative : Chem. Commun., 6540-6542 (2008)
